Depuis sa création en 1985, Hemodia a développé un savoir-faire de pointe en matière de dispositifs médicaux et électro-médicaux :
- Sets de soins à usage unique
- Pompes d'arthroscopie
- Tubulures et composants polymères à usage médical
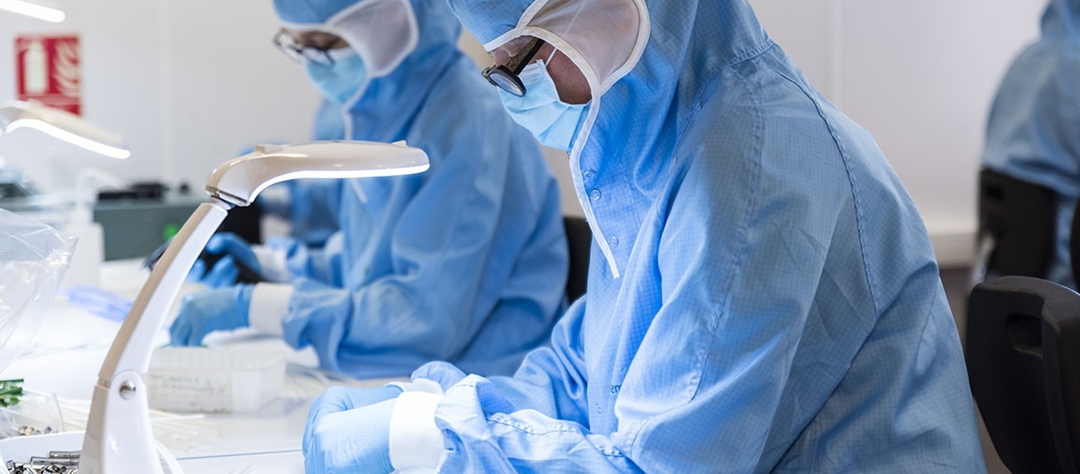
Depuis sa création en 1985, Hemodia a développé un savoir-faire de pointe en matière de dispositifs médicaux et électro-médicaux :

A travers l'ensemble des engagements présentés, Hemodia prend en compte les impacts sociétaux et environnementaux dans ses activités.
Plusieurs objectifs de développement durable dans nos technologies, notre environnement et notre économie ont été définis pour contribuer à l'amélioration de la société et à la protection de l'environnement.

Nos équipes sont à votre disposition pour répondre à vos questions.

Hemodia est à la recherche de nouveaux talents dans de nombreux domaines.